
1 June 2023 - ESDPPP NEWS
Webinar on Therapeutic Drug Monitoring in Children
Invited Speakers:
Prof. Wei Zhao, Institute of Clinical Pharmacology, Shandong University, China
Dr. Rick Admiraal, Maxima Center for Pediatric Oncology, Utrecht, the Netherlands
Programme:
Model-informed precision dosing of antibiotics in children
TDM in paediatric oncology: past, present and future
21 February 2023 - ESDPPP NEWS
TDM Working Group
as announced on our last conference in Liverpool, all members with special interest in Therapeutic Drug Monitoring (TDM) are kindly invited to participate in ESDPPP's first working group. The first meeting will be held on Tuesday, 21st Feb (4:30-5:30pm) and aims to gather ideas and potential project with fruitful discussions. In case you are interested or want to receive more details, please directly contact our treasurer Pieter De Cock: pieter.decock@uzgent.be
7 December 2022 - ESDPPP NEWS
Webinar on AI
Speakers:
1) Matthias Schwab – introduction and moderator
2) Jolien Freriksen - Saskia de Wildt - “literature searches and AI”
3) Anke-Hilse Maitland van der Zee - topic “paediatric Asthma and AI”
4) Maaike van der Lee - “AI and drug metabolising enzymes”
5) Wei Zhao - “dose machine learning”
6) Dirk Lanzerath - “AI and ethical considerations”
17 October 2022 - ESDPPP NEWS
ESDPPP BOARD ANNOUNCES CONGRESS LOCATIONS FOR 2025 & 2027
The congresses for 2025 will be held in the Netherlands and the congress for 2027 will be held in Germany. We warmly welcome our presidents elect: Saskia De Wildt and Antje Neubert and are
looking forward to interactive, informative events.
1 July 2022 - ESDPPP NEWS
YoungESDPPP Community
During the Liverpool conference 2022 a young society within ESDPPP has been emerged with broad initiatives from president Mark Turner and president elect Pavla Pokorna. YoungESDPPP brings PhD
candidates and post docs together and facilitates informal and fruitful discussions on pediatric pharmacology. More information will be provided by Anna-Maria Wiesinger and Eva Degreauwe as
co-founders and co-managers (anna.wiesinger@pmu.ac.at).
28 July 2022 - ESDPPP NEWS
General Clinical Pharmacology Considerations for Neonatal Studies for Drugs and Biological Products - Guidance for Industry, FDA
28 February 2022 - ESDPPP NEWS
ESDPPP BOARD ANNOUNCES CONGRESS LOCATIONS
The congresses for 2022 will be held in Liverpool and the congress for 2023 will be held in Prague. We welcome your ideas and suggestions for this event.
Recently Published
Current knowledge, challenges and innovations in developmental pharmacology: a combined c4c expert group and ESDPPP White paper
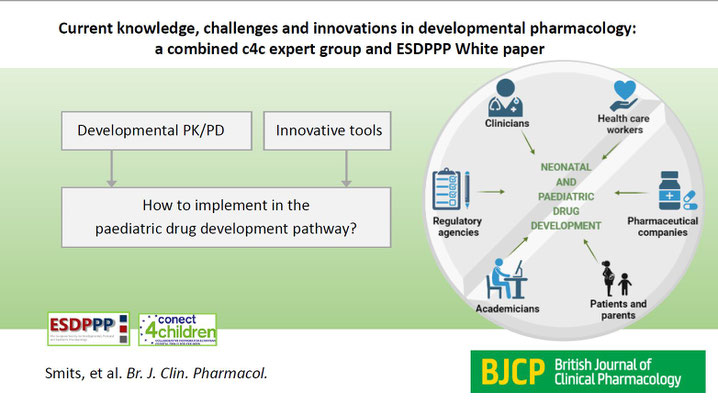

Current knowledge, challenges and innovations in developmental pharmacology: a combined c4c expert group and ESDPPP White paper
Anne Smits, Pieter Annaert, Giacomo Cavallaro, Pieter A.J.G. De Cock, Saskia N. de Wildt, Jenny M. Kindblom, Florian B. Lagler, Carmen Moreno, Paula Pokorna, Michiel F. Schreuder, Joseph F Standing, Mark A. Turner, Benedetto Vitiello, Wei Zhao, Annelie-Martina Weingberg, Raffaella Willmann, John van den Anker, and Karel Allegaert
On behalf of the Innovative Medicines Initiative conect4children (IMI c4c) Expert group on Developmental Pharmacology and the European Society for Developmental, Perinatal and Paediatric Pharmacology (ESDPPP)
Developmental pharmacology describes the impact of maturation on drug disposition (pharmacokinetics, PK) and drug effects (pharmacodynamics, PD) throughout the paediatric age range. This paper, written by a multidisciplinary group of experts, summarizes current knowledge, and provides suggestions to pharmaceutical companies, regulatory agencies and academicians on how to incorporate the latest knowledge regarding developmental pharmacology and innovative techniques into neonatal and paediatric drug development.
Biological aspects of drug absorption, distribution, metabolism and excretion (ADME) throughout development are summarized. Although this area made enormous progress during the last two decades, remaining knowledge gaps were identified. Minimal risk and burden designs allow for optimally informative but minimally invasive PK sampling, while concomitant profiling of drug metabolites may provide additional insight in the unique PK behavior in children. Furthermore, developmental PD needs to be considered during drug development, which is illustrated by disease- and/or target organ-specific examples. Identifying and testing PD targets and effects in special populations, and application of age- and/or population-specific assessment tools are discussed. Drug development plans also need to incorporate innovative techniques like preclinical models to study therapeutic strategies, and shift from sequential enrollment of subgroups, to more rational designs.
To stimulate appropriate research plans, illustrations of specific PK/PD-related as well as drug safety-related challenges during drug development are provided. The suggestions made in this joint paper of the Innovative Medicines Initiative conect4children Expert group on Developmental Pharmacology and the European Society for Developmental, Perinatal and Paediatric Pharmacology, should facilitate all those involved in drug development.
Smits, et al. Br. J. Clin. Pharmacol. (accepted) - Contact: anne.smits@uzleuven.be
25.6.2021 - WEBINAR
CSPT - ESDPPPP Joinde WEBINAR: Studying Rare Diseases

Login Details upon personal invitation of members.
14 October 2020 - WEBINAR
ESDPPPP WEBINAR: PAEDIATRIC PHARMACOLOGY OF COVID-19
The first of the new ESDPPP series of webinars will be held on 16 November 2020, focused on the following learning points:
- Pediatric drug therapy: which drugs and how did this evolve over time.
- How model-based doses can support optimal dosing in children.
- Pragmatic approach to collect PK and PD data in the real-world clinical setting.
28 August 2020 - PUBLICATION
A JOINT POLICY STATEMENT BY THE EAP AND THE ESDPPP ON OFF-LABEL USE OF MEDICINES IN NEONATES, INFANTS, CHILDREN, AND ADOLESCENTS WAS PUBLISHED EARLIER THIS
YEAR
The availability of medicines with an appropriate label for pediatric use is lagging behind those for adults, and even available pediatric drugs are sometimes not suitable to administer to children. Consequently, health-care professionals often have no other option than to prescribe off-label medicines to children. However, off-label use of medicines is in general not supported by the same level of evidence as medicines licensed for pediatric use.
09 July 2020 - WEBINAR
CLINICAL LACTATION STUDIES AND THE ROLE OF PHARMACOKINETIC MODELING AND SIMULATION IN PREDICTING DRUG EXPOSURE IN BREASTFED INFANTS
As part of the 2019-2020 Sumner J. Yaffe Lecture Series a webinar will be held on July 30 about 'Clinical Lactation Studies and the Role of Pharmacokinetic Modeling and Simulation in Predicting Drug Exposure in Breastfed Infants'.
08 May 2020 - COVID-19 INFORMATION
UPDATED REVIEW ON AZITHROMYCIN FOR COVID-19 IN PREGNANCY
Azithromycin has been increasingly included in the treatment regimens of COVID-19. An updated review published in the Motherisk International Journal, looks at whether azithromycin poses a risk to the fetus and whether it is compatible with breastfeeding.
25 April 2020 - COVID-19 INFORMATION
IUPHAR's RESPONSE TO COVID-19
IUPHAR is responding to the current crisis by coordinating pharmacological resources worldwide. Although most of the current research is done in adults, we believe in part it may be relevant for pediatric pharmacologists as well.
20 February 2020 - EVENT INFORMATION
NEONATAL THERAPY 2020: LATEST ADVANCES AND INSIGHTS
Neonatal Therapy 2020 is a one-day course on the latest ideas and innovations to improve neonatal pharmacological therapy, organised by the ESPR Pharmacology Section and endorsed by the European Society for Paediatric Research (ESPR) and the Academic Paediatrics Association (APA).
9 July 2018 - ANNOUNCEMENT
FAREWELL SYMPOSIUM PROF. DR. DR. WOLFGANG RASCHER
At September 29th, 2018, the farewell symposium for Prof. Dr. Dr. Wolfgang Rascher will be held at Universitätsklinikum Erlangen, Department of Paediatrics and Adolescant Medicine. Several ESDPPP members will be present both as speakers and as participants.
8 January 2017 - FROM THE EUROPEAN UNION
EUROPEAN COMMISSION LAUNCHES A PUBLIC CONSULTATION ON THE PAEDIATRIC REGULATION
The European Commission (EC) has launched a public consultation to get views and feedback from stakeholders, to support the Commission in drafting its second report on the Paediatric Regulation
after nearly ten years of implementation. The consultation is open until 20 February 2017.
15 February 2016 - NEWSLETTER
LATEST ISSUE OF PHARMACOLOGY INTERNATIONAL
Latest issue of Pharmacology International The latest issue of “Pharmacology International”, the International Union of Basic and Clinical Pharmacology (IUPHAR) newsletter, is now available at
http://www.iuphar.org/files/Newsletters/Pharmacology_International_2015_December.pdf. We would appreciate if you would forward this e-mail to all of your members or post the newsletter PDF, or a
link to it, on your society website. Highlights […]
15 January 2015 - ESDPPP NEWS
EXPLORATORY CLINICAL STUDIES IN CHILDREN: OBTAINING PRELIMINARY DATA USING SUB-THERAPEUTIC OR SUB-PHARMACOLOGIC DOSES
Exploratory clinical studies in children The MRC Centre for Drug Safety Science (CDSS) at the University of Liverpool has organised a scientific workshop (agenda attached) entitled “Exploratory
clinical studies in children: obtaining preliminary data using sub-therapeutic or sub-pharmacologic doses”, to be held in Liverpool on Thursday 12th February 2015. The workshop will focus on
innovative […]
14 November 2014 - ESDPPP NEWS
ESDPPP BOARD ANNOUNCES CONGRESS LOCATIONS
The congresses for 2017 to 2021 will be held in Leuven, Basel and Liverpool. We are welcoming your ideas and suggestions for these events.
7 May 2013 - ESDPPP NEWS
NEWSLETTER ARTICLE ON 25 YEARS OF ESDPPP
The ESDPPP celebrates its 25th anniversary in 2013. This milestone provides an important opportunity to celebrate ESDPPP’s achievements to date and to describe our future aspirations and
ambitions on behalf of millions of paediatric patients worldwide. Download report
Contact information
ESDPPP VZW p/a University Hospital
Gasthuisberg Herestraat 49
B-3000 Leuven

